What Is Telomere And Why Does Telomere Length Matter?
Science • March 23, 2022 • 14min read
Have you ever thought of living forever?
It may seem impossible. But, did you know that we have molecules inside our cells that can make cells immortal? So, why is not everyone centenarian? Why do we age? We are going to introduce the telomere – the DNA region at the tip of our chromosomes, which is very famous in the anti-aging and longevity field. Also, we are going to talk about how telomeres contribute to long life.
What Is A Telomere?
Telomeres are special DNA structures that are only found at the ends of chromosomes, the highly-condensed form of the DNA inside the cells. Humans have 46 chromosomes in each cell, wherein all of their tips are capped with telomeres. The function of telomeres is to protect the DNA from damage. Telomeres in humans are made up of long continuous TTAGGG repeats at the end of the chromosome, normally repeating 3,000 times and are up to 15,000 DNA nucleotides long. The word telomere comes from the Greek words telos (meaning “end”) and meros (meaning “part”).
Telomere lengths in human chromosomes vary dramatically. Depending on the kind of tissue, the age of the person, the chromosome, and the number of replications the cells already had, telomeres may have around 500 to 15,000 DNA nucleotides composed of TTAGGG repeats.
Telomeres safeguard chromosomal ends from degradation and fusion with other chromosomes when the cells divide to create more cells. As an example, we lose around 30, 000 to 40, 000 dead skin cells every minute or around 50 million cells every day. Hence, skin cells are constantly dividing to replenish the lost cells. During the process of cell division, the cell must fully replicate the genetic information first before it divides into two identical cells.
However, every time the DNA is replicated, the telomere DNA nucleotides (TTAGGG repeats) at the end of the chromosome are lost.
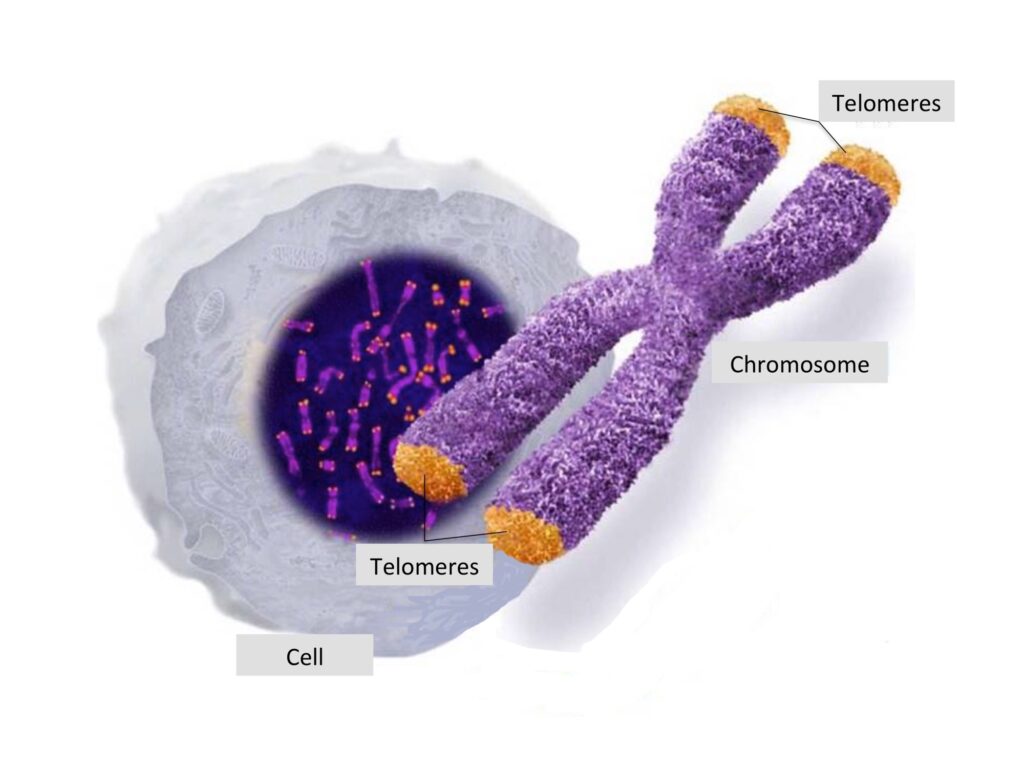
Telomeres are DNA structures at the end of chromosomes. Image source
Why Do Telomeres Shorten?
To prevent cells from losing important genetic information, telomeres are there at the end of chromosomes, like the protective caps in shoelaces, to prevent them from fraying or getting worn out during the DNA replication process. This way, the telomeres shorten while leaving the critical part of the DNA undamaged. If telomeres were not present, the important regions of DNA that code for genes would be lost every time a cell divides, leading to loss of genetic information, then causing drastic consequences such as cell death or diseases.
Unfortunately, there is an issue with the DNA copying mechanism of cells. The end of each chromosome cannot be replicated. As a result, after every DNA replication, telomeres get shorter. This problem is known as the “end-replication problem”, caused by the inability of the DNA polymerase (the enzyme that catalyzes DNA replication) to fully copy the DNA at the end of the sequence, resulting in a loss of roughly 20-200 nucleotides with every cell division.
When the chromosome telomere reaches a critical length where it gets too short to be further duplicated, the limit on the multiplication of cells is reached. This is known as Hayflick’s limit which estimates that a human cell can only divide 40-60 times. Then the cell enters a state of senescence (a state where the cell does not divide anymore) or undergoes apoptosis (a cell suicide process which is also known as programmed cell death).
These mechanisms are believed to prevent the cells from passing the damaged or abnormal chromosomes to the new cells or prevent them from developing into cancer cells.
Meanwhile, the rate of telomere loss is also influenced by oxidative stress, a phenomenon where there’s a cellular accumulation of free radicals such as reactive oxygen species (ROS) that directly damages the DNA.
Lifestyle variables such as food, smoking, and stress are considered to affect the level of oxidative stress in the body and thus are important factors in telomere length. Besides, telomere length shortening is also accelerated at the onset of various diseases, particularly those related to aging and degenerative diseases.
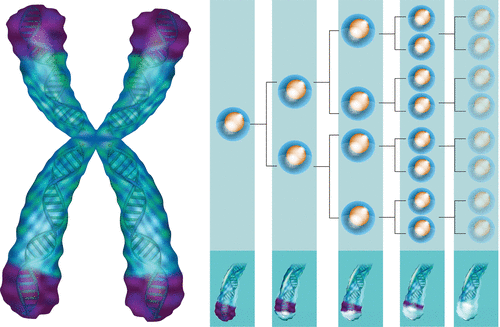
Telomeres’ length shortens with each cell division. Image source
Telomeres And Aging
Telomere has been in the spotlight ever since its association with aging and its role in cell longevity were discovered. Scientists are still attempting to unravel the issue of why we age or what causes aging. And according to one theory, our telomeres are at least partially responsible for our aging process.
So, what telomere length is normal or good for people? Telomeres in newborns typically range in length from 8,000 to 13,000 nucleotides. Each year, this number decreases by around 20-40 nucleotides.
So, by the time a person reaches the age of 40, telomeres might have already lost up to 1,600 nucleotides. In elderly people, telomere measurement showed that they still have around 5, 000 nucleotides left in the telomeres.
Interestingly, telomere length is not the only factor to consider that impacts the rate of the aging process. Aside from length, the form and structure of telomeres are also important aging modulators.
At the ends of the chromosomes, healthy telomeres normally form tiny paperclip-shaped loops, tucking the telomere ends away and keeping them secure. This is done by the shelterin complex, a molecule that binds the telomere to form and stabilize the loop to prevent the very end of the telomeres from getting exposed.
Otherwise, if the tips of telomeres are exposed instead of being tucked into the supposed loop structure, it can indicate the presence of broken DNA. Also, if the telomere tips are exposed, they can be recognized by the cell’s DNA repair system as damaged DNA that needs to be corrected. Hence, without shelterin, telomeres will become unstable, cell division will end, and eventually, senescence or apoptosis will be triggered.
In general, people with shorter telomeres have a higher mortality rate than those with longer telomeres. Excessive or rapid telomere shortening may have a variety of consequences for one’s health, and having longer telomeres than average is also associated with fewer diseases and having a longer lifespan.
Telomeres And Age-related Diseases
Although there is still controversy about how telomere biology impacts lifespan, a correlation has been shown between telomere length and mortality. Multiple studies also have shown links between telomere length and age-related disorders such as cancer, cardiovascular disease, and neurological diseases which have significant impacts on human life duration.
Cancer development
In humans, cancer has a detrimental impact on life expectancy. Both very short and very long telomeres are found in cancer development and survival. When the telomeres are short, the DNA information cannot be properly protected and DNA damage or breakage may lead to the development of tumors. Conversely, when telomeres are very long, the cancer cells may survive because the telomere protects them from triggering the death of cancer cells.
80-90% of all tumors have activated telomerase – many cancer types take advantage of telomere length to maintain their immortality by abnormal telomerase activity.
The telomerase is a protein molecule that adds more TTAGGG repeats to the chromosome ends to prevent telomere shortening. This process is usually inactive in our body cells, but it is active in our stem cells so that it can continuously produce new cells throughout our lives.
Cancer activates the telomerase enzyme to keep adding more telomere repeats and prevent telomere shortening, hence escaping the cell death program of Hayflick’s limit and making them immortal. This is why, although we have the built-in mechanism to make our cells immortal, these cells have the potential to become cancerous after a while.
Cardiovascular diseases
Cardiovascular diseases, together with cancer, are the leading causes of illness in the aging population. Diabetes, as well as insulin resistance alone, for example, has been linked to a reduction in telomere length.
Telomeres have also been linked to the development of hypertension and atherosclerosis. Particularly, people with normal blood pressure and with short telomeres are more likely to contract hypertension. Meanwhile, hypertensive people with short telomeres are more likely to develop atherosclerosis.
Telomere shortening was also observed in atherosclerosis, primarily during the early stages of coronary artery heart disease. Several studies have associated the cells found in atherosclerotic plaque with shorter telomeres.
The cells in the artery, which are the most vulnerable to atherosclerosis are often stressed by high arterial pressure, cholesterol deposition, and oxidative stress. According to several clinical investigations relating coronary artery disease to telomere length, the telomeres of circulating white blood cells in patients with coronary artery disease are around 300 nucleotides shorter than in controls which is equivalent to a nearly 9-year age difference. Surprisingly, the kids of patients with coronary artery disease had shorter telomeres compared to the offspring of parents who do not have atherosclerosis.
Another disorder is chronic heart failure (CHF) which is also a common cardiovascular diagnosis across the globe and is linked with a significant mortality rate. Although the incidence and frequency of CHF may rise sharply with age, there is a wide range of factors explaining the disease susceptibility, onset age, and pathogenesis severity. This variation cannot be entirely attributable to the existence of traditional risk factors, and new data suggest that telomere biology may play a role.
Endomyocardial biopsies from heart failure patients have shown that their heart cells had shorter telomeres, a higher degree of cellular senescence, and cell death. In addition, the severity of heart failure was likewise linked to the telomere length. Renal function impairment was also found to be linked to a poorer prognosis in CHF patients.
Alzheimer’s Disease
Alzheimer’s disease (AD) is a slowly progressive neurological disease that is a leading cause of dementia in the elderly across the globe. In patients with Alzheimer’s disease, telomere shortening and a decrease in telomerase activity have been observed.
Clinical investigations have shown that Alzheimer’s patients had considerably shorter telomeres, implying that telomere attrition and conversion from moderate cognitive impairment (MCI) to AD are linked. In this disease, oxidative stress may cause persistent inflammation and contribute to neuronal malfunction, telomere shortening, and brain cell death.
When researchers looked at telomeres in brain tissues, they identified a clear link between telomeres in leukocytes and the cerebellum, with AD patients’ leukocytes and cells being considerably shorter than controls.
Parkinson’s Disease
Parkinson’s disease is characterized by neurodegeneration cell loss and has both motor and non-motor symptoms which are also linked to chronic inflammation and oxidative stress.
Telomere shortening appeared to be linked to plasma carbonyl protein concentrations in Parkinson’s patients. The carbonylation of proteins is one of the causes of oxidative stress in PD. It is the abnormal addition of extra chemical groups called ‘carbonyl’ (composed of a backbone of carbon and oxygen atoms) – that is induced by ROS (reactive oxygen species).
This event promotes irreversible and severe oxidative damage that impairs the function of proteins in cells leading to abnormalities. Usually, these abnormal carbonylated proteins are degraded by the cell, but cannot be eliminated if they are heavily carbonylated.
Through time, these carbonylated proteins form aggregates and their accumulation in tissues has been directly observed in a large number of neurodegenerative diseases including PD.
DNA damage is thought to cause gradual hypomethylation at DNA repeat sites and is correlated with shorter telomeres. However, research on Parkinson’s disease patients had varying results. Some studies have linked shorter telomeres to a lower risk of Parkinson’s disease.
Yet, in certain studies, telomeres were shown to be significantly shorter in older PD patients in their 50s and 60s. These inconsistent findings are unsurprising given the lack of research on telomeres and Parkinson’s disease. To get a more comprehensive conclusion, additional research with a bigger cohort is required.
Is Telomere Length A Reliable Biological Age Predictor?
Since telomere length shortens with every cell division throughout life, it was found to be inversely correlated with age – the shorter the telomere, the older the age a person is.
However, this isn’t the only biological indicator of aging. Cell aging is also influenced by oxidative stress. The degree of oxidative stress in the body is thought to be influenced by lifestyle factors such as diet, smoking, and stress.
Longer telomeres may not always imply a longer lifespan. There is still a lot to understand before we can declare it a primary cause of aging. There are controversial opinions among scientists if telomere shortening is a direct cause of aging or just an external sign of becoming older. However, we do know that some unhealthy lifestyle choices may hasten telomere shortening, therefore we should take steps to slow it down.
How To Restore Telomere Length?
Telomerase is a specialized enzyme that adds the TTAGGG telomere sequence to chromosomal ends. Our telomeres are built up before we are born, and then maintained throughout our lives, due to this synthesis of new DNA at the ends of the chromosomes.
Telomeres are naturally reduced when genetic information is copied and cells divide. In normal cells, the telomeres shrink as a result of the lack of telomerase to generate more DNA repeats, until they become dangerously short and trigger cell senescence or apoptosis.
In most human cells, telomerase is missing or present at extremely low levels. The restriction of telomerase is hypothesized to be a method for limiting cell growth. This may serve as an anti-tumor defense mechanism.
Limitations in telomerase activity in the adult body, which cannot compensate for the increasing telomere shortening that occurs when cells divide during tissue regeneration, are thought to be the cause of telomeric shortening during aging. The loss of telomeres over time is thought to contribute to organismal aging.
Cells can no longer divide properly when their telomeres grow extremely short. A variety of telomere biology diseases (TBDs), also known as short telomere syndromes or telomeropathies, may arise when this condition is systemic.
The presence of dangerously short telomeres defines a set of illnesses, which have a wide range of symptoms and causes. TBD-related consequences may occur as a result of illnesses, which are commonly observed in highly proliferative cells. These consequences present symptoms such as bone marrow failure negatively affecting the stem cells.
Meanwhile, diet and nutrition, exercise, stress, smoking, and obesity, are all culprits in telomere shortening. Hence, choosing balanced nutrition and making healthy choices can help to maintain steady and functional telomeres.
Does NMN Supplementation Increase Telomere Length?
While research into quantifying biological aging in people is still in its early stages, it seems to be promising. Anti-aging research has also provided hope to people who desire to delay or even reverse the aging process. Anti-aging research is mostly focused on improving the availability of a chemical called Nicotinamide adenine dinucleotide (NAD+) to cells.
According to studies, the amount of NAD+ accessible to the body decreases as people age. Because NAD+ is a helper molecule with a variety of roles in the body, the most significant of which is activating Sirtuins to control metabolism.
The loss of NAD+ in the body as people become older is linked to a variety of age-related disorders, including cancer, cardiovascular, and neurodegenerative diseases.
NAD+ can also alleviate the negative effects of telomere dysfunction. More evidence demonstrates that telomeres are also modulated by the longevity genes – Sirtuins.
SIRT1 was also found to be involved in maintaining telomere length, and further, a mice study demonstrated that SIRT1 overexpression may increase telomere length as well. But since SIRT1 is NAD-dependent, NMN supplementation can support healthy maintenance of telomere length.
Strikingly, a 2021 study published in Frontiers in Nutrition has also demonstrated oral NMN supplementation extends the telomere length in mice and humans, suggesting an effective strategy as an anti-aging pill.
Closing Remarks
Indeed, telomeres can make cells immortal – escaping cell death with the help of telomerase. The mechanism behind it is complex and it is not simple to manipulate it to make our cells immortal.
But if only scientists can figure out a strategy to manipulate telomere length without triggering the undesirable effects, maybe we can do the same with our cells.
Yet, it may not be possible now, but in the near future – breakthrough discoveries in telomeres and telomerase biology may bring us closer to living an even longer and more fulfilling life. Who knows, in the future, pills may be available in a pharmacy for extending our lives – or our telomeres, rather – for a year, a decade, or another century.